Spinal Cord Stimulators
Spinal cord stimulators were approved by the Food and Drug Administration (FDA) in 1989. They were found to be extremely useful for patients who experienced chronic pain in their backs and limbs and failed to get relief from other methods or treatments. Today, with the advancements in technology, successful patients report a 50% to 100% relief from pain, through neurostimulation therapy.
About Spinal Cord Stimulation
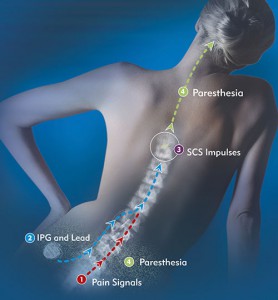 Spinal Cord Stimulation, or SCS, may offer hope for many of the estimated 100 million people who suffer from chronic pain.1 SCS sends electrical impulses that trigger nerve fibers along the spinal cord, masking the pain message traveling to the brain. When this happens, the painful sensation is replaced with a soothing, tingling sensation.
Spinal Cord Stimulation, or SCS, may offer hope for many of the estimated 100 million people who suffer from chronic pain.1 SCS sends electrical impulses that trigger nerve fibers along the spinal cord, masking the pain message traveling to the brain. When this happens, the painful sensation is replaced with a soothing, tingling sensation.
SCS may be prescribed for chronic intractable pain of the trunk and/or limbs, including unilateral or bilateral pain associated with the following:
- failed back surgery syndrome
- intractable low back pain and
- leg pain.
Many people with failed back surgeries have had success with the SCS. It is most commonly used to treat low back and lower extremity pain. Thousands of patients with severe chronic painful conditions have received relief with spinal cord stimulation.1
What is a Spinal Cord Stimulator
How Does It Work
Generally speaking, neurostimulation works because it blocks the pain signal from reaching the brain.
Through the application of an electrical current, a soft stimulation is created that blocks the brain’s ability to sense pain from the neck, lower back, legs, hips or arms. This is accomplished by one or two ‘leads’ that are placed into the back through a needle. A small incision is made and a tiny, programmable generator is placed in the upper buttock and flank area, under the skin, which generates electrical currents to the leads, which offset the sense of pain.
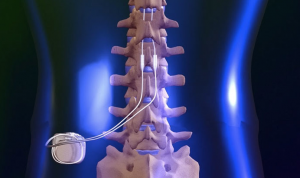
After the “test drive”, the patient and physician decide whether or not spinal cord stimulation is a therapy option. If they decide to go forward, then the patient will undergo another surgical procedure to surgically place the Precision™ Implantable Pulse Generator. The leads may be inserted in a procedure similar to an epidural. In some cases, a physician may recommend a surgical lead, also known as a paddle lead. In this case, the paddle lead is placed at the target site during the surgical procedure. Patients may remain awake during this procedure, under local anesthesia and light sedation. In other cases, general anesthesia may be administered.
Besides a cordless remote control, the patient will also receive a portable, cordless charger and a base station for the charger.
NeuroSpine doctors have had great success in eliminating the chronic pain that many patients have lived with for years. Our doctors use several different spinal cord stimulator manufacturers, depending on what the doctor believes is the best fit for the patient.
Trudi’s Story | Spinal Cord Stimulator Testimonial
1Institute of Medicine Report Brief
The Boston Scientific Neuromodulation Spinal Cord Stimulator (SCS) Systems are indicated as an aid in the management of chronic intractable pain of the trunk and/or limbs, including unilateral or bilateral pain associated with the following:
failed back surgery syndrome, intractable low back pain, and leg pain.
Precision Spinal Cord Stimulator System please refer to the Precision Spinal Cord Stimulator System Clinician Manual.
